Abstract
Introduction Chronic neutropenia (CN) encompasses different blood disorders all characterized by low levels of neutrophils (absolute neutrophil count [ANC] <1500/µl for >3 months) and is associated with increased risk of recurrent and/or severe infections [Donadieu J. Exp RevHematol. 2021]. The most common chronic neutropenic disorders (CNDs) are chronic idiopathic neutropenia (CIN), which is not attributable to drugs or a specific infection or inflammatory, malignant, or genetic cause [Dale DC. Curr Opin Hematol. 2017]; cyclic neutropenia (CYN), characterized by recurrent episodes of neutropenia approximately every 21 days [Donadieu J. Exp Rev Hematol. 2021]; and severe congenital neutropenia (SCN), caused by genetic variants [Skokowa J. Nat Rev Dis Primers. 2018]. The current management strategy for CN is long-term treatment with injectable granulocyte colony-stimulating factor (G-CSF). Although typically effective in the short-term, G-CSF comes with many adverse effects such as bone pain and, especially for patients with SCN, an increased risk for hematologic malignancy [Newburger PE. Semin Hematol. 2013; Dale DC. Blood Advances. 2022]. Mavorixafor is an investigational oral antagonist of CXCR4 in clinical development for treatment of patients with multiple CNDs, including Warts, Hypogammaglobulinemia, Infections, and Myelokathexis (WHIM) syndrome [Dale DC. Blood. 2020]. Herein we report results of phase 1b, open-label, multicenter trial (NCT04154488) evaluating the safety, tolerability, and serving as a proof of concept for efficacy, of mavorixafor alone or with concurrent G-CSF use across several CNDs.
Methods Patients aged ≥12 years with diagnosis of CIN, congenital neutropenia, or CYN ≥6 months prior and ANC <1000/µL at screening visits were included. Patients on G-CSF with ANC >1000/µL were also included. All patients with congenital neutropenia without documented genetic variants underwent genetic screening via a 642-gene comprehensive immune and cytopenia panel. Patients received ≥1 dose of mavorixafor (>50 kg, 400 mg; ≤50 kg, 200 mg), regardless of current treatment with or without G-CSF. Patients were monitored for hematologic parameters such as total white blood cell (WBC) and subset counts, including ANC, absolute lymphocyte count (ALC), and absolute monocyte count (AMC), over the course of 6 to 8 hours 1 day before receiving mavorixafor (day -1) and on the first day of mavorixafor treatment (day 1) and were followed for 30 days for safety and tolerability. Fold change in total peripheral WBC count, ANC, ALC, and AMC from day -1 levels was assessed.
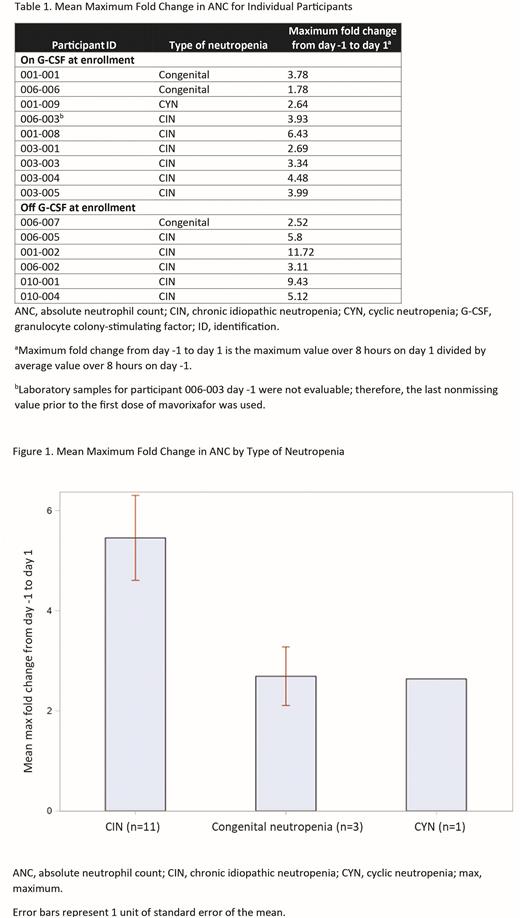
Results As of June 2022, 15 patients (CIN, n=11; congenital neutropenia, n=3; CYN, n=1) were enrolled and dosed. The mean (SD) age in years was 39.7 (17.2); 40% were male, and 60% were on G-CSF. All patients received ≥1 dose of mavorixafor once daily. The mean (SD) maximum fold change in ANC from day -1 to day 1 across all included patients was 4.72 (2.74) at peak over the course of 6 to 8 hours after dose 1. Table 1 shows fold change in ANC for individual participants, and Figure 1, by type of neutropenia. The mean (SD) maximum fold change in other hematologic parameters, including total WBC count, ALC, and AMC, from day -1 to day 1 was 3.14 (0.65), 3.05 (0.62), and 2.64 (0.80), respectively. For the subgroup of patients on G-CSF at day -1, the mean (SD) maximum fold change in total WBC count, ANC, ALC, and AMC from day -1 to day 1 was 2.87 (0.58), 3.67 (1.29), 3.07 (0.73), and 2.44 (0.59), respectively. For patients not on G-CSF, the mean (SD) maximum fold change in total WBC count, ANC, ALC, and AMC from day -1 to day 1 was 3.54 (0.52), 6.28 (3.45), 3.01 (0.47), and 2.95 (0.95), respectively. Treatment-emergent adverse events (AEs; n=11) were all grade 1; no serious AEs were reported.
Conclusions Meaningful increases in ANC and all other hematologic parameters evaluated were observed on day 1 in all patients treated with ≥1 dose of mavorixafor. Responses were observed regardless of concurrent G-CSF use or type of CN. Mavorixafor treatment was well tolerated overall, with only mild AEs reported. This study supports the potential therapeutic use of mavorixafor in the treatment of CNDs beyond WHIM syndrome and underscores the possibility for mavorixafor to become the first oral treatment for CN.
Disclosures
Warren:X4 Pharmaceuticals, Inc.: Consultancy. Walkovich:Sobi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pharming: Membership on an entity's Board of Directors or advisory committees; X4 Pharma: Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Horizon: Membership on an entity's Board of Directors or advisory committees; NICER Consortium: Other: Executive Chair; St. Jude: Honoraria; American Society of Hematology: Honoraria; UpToDate: Patents & Royalties. Bolyard:X4 Pharmaceuticals, Inc.: Research Funding. Dickerson:Bluebird Bio: Membership on an entity's Board of Directors or advisory committees. Walter:Enzyvant: Consultancy; Grifols: Consultancy; CSL-Behring: Consultancy; X4 Pharmaceuticals, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees; Octapharma: Research Funding; ADMA Biologicals: Consultancy; MustangBio: Research Funding; Regeneron: Consultancy; UptoDate: Other: Medical Writer; Pharmig: Membership on an entity's Board of Directors or advisory committees; Chiesi: Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Cadavid:X4 Pharmaceuticals, Inc.: Current Employment, Current equity holder in publicly-traded company. Chapa:X4 Pharmaceuticals, Inc.: Consultancy. Chen:X4 Pharmaceuticals, Inc.: Current Employment, Current equity holder in publicly-traded company. MacLeod:X4 Pharmaceuticals, Inc.: Current Employment, Current equity holder in publicly-traded company. Peters:X4 Pharmaceuticals, Inc.: Current Employment. Polisson:X4 Pharmaceuticals, Inc.: Consultancy. Dale:X4 Pharmaceuticals, Inc.: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal